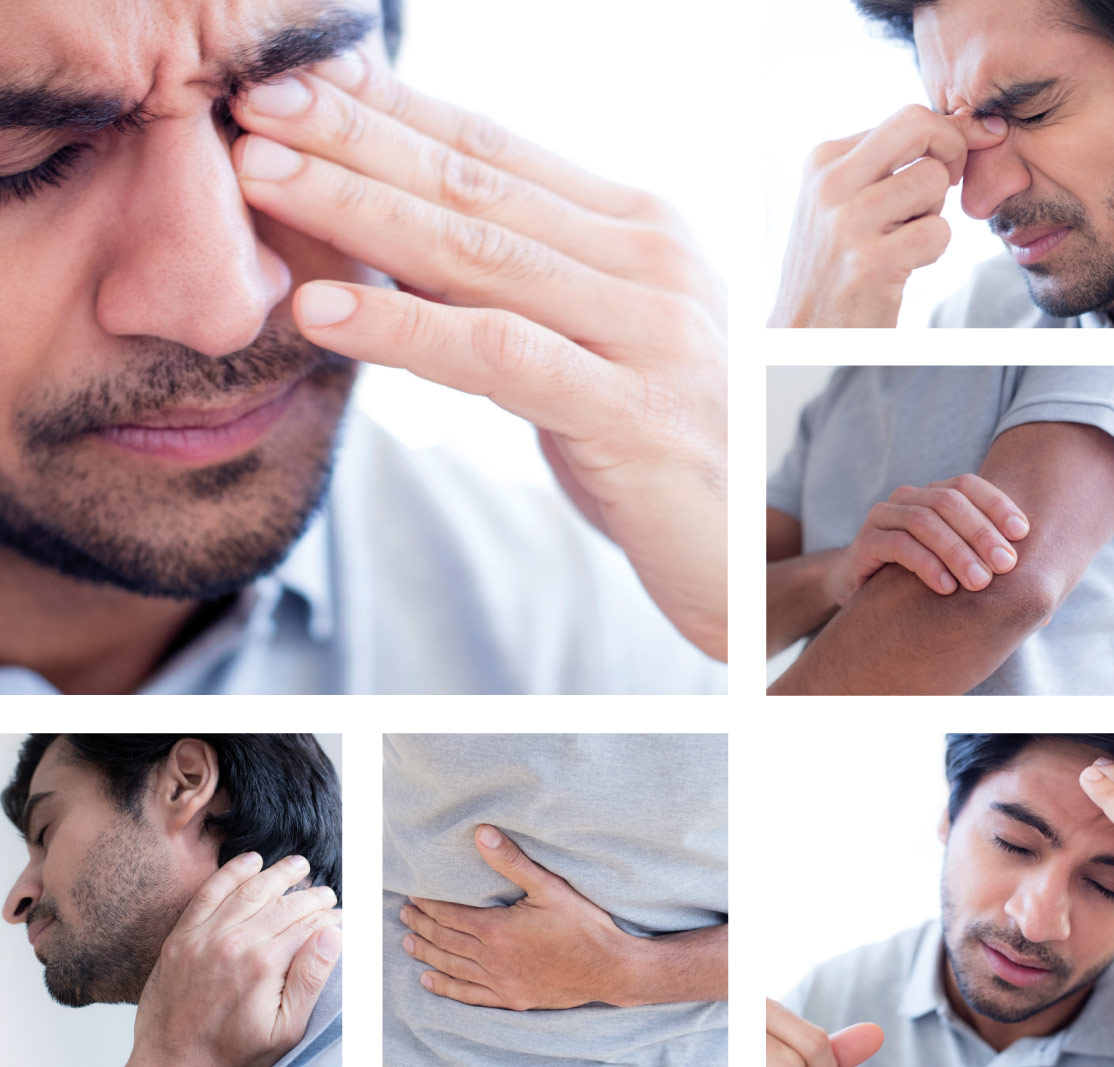

The symptoms of PAI are mostly non-specific, multiple and varied.2 They include, but may not be limited to:2-5
Undiagnosed, PAI may progress to an adrenal crisis. The signs and symptoms include, but may not be limited to the following:2
Preparation should be made in advance to combat any anaphylactic reaction that may occur after an injection of Synacthen. In the event of a serious anaphylactic reaction, the patient should be treated appropriately with adrenaline and steroids.1
Synacthen Ampoules should not be used in the presence of active infectious or systemic diseases, when the use of live vaccine is contemplated or in the presence of a reduced immune response, unless adequate disease specific therapy is being given.1
Administration should be under the supervision of appropriate senior hospital medical staff with observation for 30 minutes after injection for signs of hypersensitivity.1

Patient's blood sample is collected
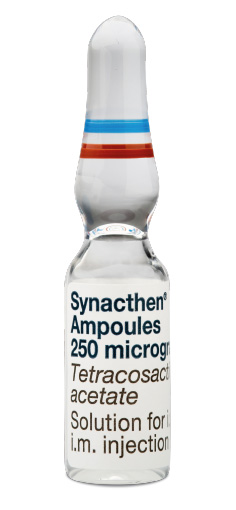
Synacthen is immediately administered via IM or IV injection

A second blood sample is taken exactly 30 minutes post Synacthen administration

In the hospital laboratory, plasma cortisol levels from both blood samples are measured and compared
Dosing: Adults: IV or IM, 250 μg (1 ml); Children: IV, 250 μg/1.73 m2 body surface area. Therefore for children aged 5 to 7 years, approximately half the adult dose will be adequate. For more accurate dosing of other ages, standard body surface area tables should be consulted1
The difference between the two plasma cortisol levels needs to be assessed.1 As can be seen below, the absolute plasma cortisol level indicates normal adrenocortical function if the second sample level is >500 nmol/litre (180 μg/litre).
Alternatively, the plasma cortisol increment indicates normal adrenocortical function if the second cortisol level has increased by ≥200 nmol/litre (70 μg/litre) compared to the first.
Any other response may be suggestive of Primary Adrenal Insufficiency1.
The predictive value of the SST in excluding PAI is approximately
The sensitivity of the SST is
(frequency unknown)1
Endocrine disorders:1 Adrenal haemorrhage
Immune system disorders:1 Hypersensitivity
(frequency unknown)1
Adverse events should be reported. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellowcard in the Google Play or Apple App Store. Adverse events should also be reported to ATNAHS Pharma UK Ltd on 00 44 (0) 1279 406759, or by email to athnahspv@diamondpharmaservices.com